
The head of the laboratory is Galina G. Belozerskaya, Doctor of Medical Sciences
Since 1986, the Laboratory of Pathology and Pharmacology of Hemostasis has been conducting research in two areas: the development of new hemostatic agents of local and systemic action and the determination of anticoagulant and antithrombotic activity.
During the laboratory’s work into research of new hemostatic agents of local and systemic action, the staff developed various methods for assessing the hemostatic activity of new drugs on an experimental model, which were approved by the Pharmaceutical Committee of the Ministry of Health of the Russian Federation for preclinical assessment of the hemostatic effect of potential drugs and described in the section "Methodological recommendations for study of drugs that affect hemostasis" in the "Guidelines for conducting preclinical studies of drugs" part one, 2012, pp. 453-479, published under the auspices of the Ministry of Health of Russia.
Laboratory staff took part in the development of the composite hemostatic material "Coletex-Gem,” which stops capillary-parenchymal bleeding in emergency rooms, burn centers, and during plastic surgery. They also participated in the development of the collagen-platelet hemostatic sponge "Trombokol,” which is used in any type of surgical intervention, since it can be left in the wound, the development of the hemostatic dressing "Hemotex", which has been widely used in emergency care and surgical practice. Coletex-Gem, Trombokol, and Hemotex are approved by the Ministry of Health and Social Development of the Russian Federation for clinical use and are produced by the Belkozin Plant, Altex + LLC, Textilprogress LLC.
Laboratory staff participated in the creation of a new preparation of recombinant factor VIIa. A comparative analysis of the hemostatic activity of “NovoSeven” with the new domestic substance rFVIIa administered intravenously was carried out, which revealed the proximity of their specific pharmacological activity. The drug has passed clinical trials and has been introduced into healthcare practice.
Together with the Laboratory of Protein Chemistry of the State Research Institute of Genetics, peptide derivatives containing secondary lysine amides were studied. The peptides For-Ala-Phe-Lys-Mf9NCl and For-Ala-Phe-Lys-Pip9NCl significantly slowed down the XIIa-dependent euglobulin lysis and lengthened the clot dissolution time compared to the control. Comparative analysis of the hemostatic effect of the studied peptides with known inhibitors of fibrinolysis (epsilon-ACA and tranexamic acid) in experimental hemorrhage showed the superiority of the peptides over ε-ACA and tranexamic acids. Patent No. 2341532 dated 20.12.2008 was obtained on this subject.
The laboratory was the first to study the effect of the physicochemical properties of substances in various dosage forms (powder, sponge and knitted materials) and their ability to stop bleeding. A clear dependence of these substances on the indicators of the adsorbing effect and moisture absorption parameters was revealed, which is of great importance for the further development of new local hemostatic agents.
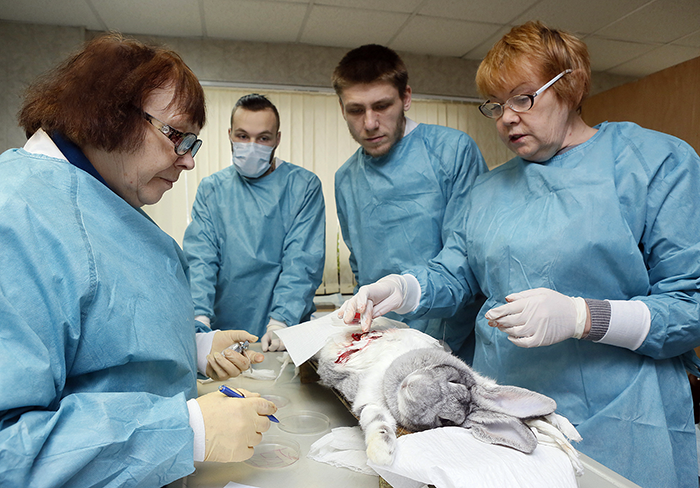
Evaluation of the hemostatic activity of new substances of local and systemic action under experimental conditions. From left to right: L.S. Malykhina, M.S. Mironov, D.Y. Bychichko, G.G. Belozerskaya
Laboratory staff, together with the Research Institute of Textile Materials, are involved in the creation of a new local hemostatic agent based on oxidized cellulose (dialdehyde cellulose), which undergoes biodegradation, making it possible to leave a hemostatic agent on the wound surface of any organ until it is completely absorbed, ensuring a quick stop of bleeding. This development is funded by a grant from the Ministry of Education and Science within the framework of the federal program "Development of the pharmaceutical and medical industry of the Russian Federation for the period up to 2020 and beyond."
Currently, together with the staff of the A.E. Favorsky Irkutsk Research Institute of Chemistry of the Siberian Branch of the Russian Academy of Sciences, the development and study of new local hemostatic agents based on metal salts of polyacrylic acid is being carried out.
5 Candidate and 1 Doctoral dissertations have been defended on this subject. 9 patents have been obtained.
Laboratory staff has also carried out research under the following contracts:
- Bid of the Ministry of Education and Science No. 02.467.11.3011 Code 2006-ЖС-00.3/001.002 "Obtaining recombinant human proteins to combat massive blood loss" (2006);
- Bid of the Ministry of Education and Science No. 02.512.11.2082 code 2007-2-1.2-05-02-040 "Development of technology for obtaining genetically engineered blood coagulation factor VIIa based on eukaryotic systems" (2007-2008);
- Grant within the framework of the Russian Academy of Medical Science program "Nanotechnologies and nanomaterials in medicine for the period 2008-2015" on the topic "Development of nanomaterials intended to combat thrombosis and hemorrhage" (2008-2012);
- Russian Foundation for Basic Research grant 04-13591 “Polymetallacrylates – a new generation of hemostatics” (2009–2009);
- State contract No. 14.N08.12.0005 with the Ministry of Education and Science of the Russian Federation on the topic "Study of the specific pharmacological activity of a biodegradable hemostatic drug to stop capillary and parenchymal bleeding" (2013).
As a result, the following patents were obtained by our employees:
1. Hemostatic sponge. Aboyants R.K., Belozerskaya G.G., Istranov L.P., Istranova E.V., Makarov V.A. Patent for invention: RUS 2122867 10.12.1998.
2. Composite hemostatic material and method for its production. Makarov V.A., Belozerskaya G.G., Oltarzhevskaya N.D., Lysun N.V., Filatov V.N., Shevereva L.G. Patent for invention: RUS 2063246 10.07.1996.
3. Hemostatic dressing. Belozerskaya G.G., Vasilyeva T.S., Makarov V.A., Subbotko O.A. Patent for invention: RUS 2200583 26.03.2002.
4. Means for improving peripheral circulation. Belozerskaya G.G., Yurtov E.V., Makarov V.A., Koroleva M.Y. Patent for invention: RUS 2246934 04.03.2003.
5. Hemostatic adhesive. Belozerskaya G.G., Malykhina L.S., Makarov V.A. Patent for invention: RUS 2256448 09.03.2004.
6. Hemostatic adhesive. Belozerskaya G. G., Malykhina L. S., Makarov V. A. Patent for invention RUS 2257901 23.07.2004.
7. Hemostatic adhesive. Belozerskaya G.G., Malykhina L.S., Makarov V.A. Patent for invention: RUS 2262937 23.07.2004.
8. Hemostatic adhesive. Belozerskaya G.G., Malykhina L.S., Mezhneva V.V., Kostava V.T. Patent for invention: RUS 2270016 09.03.2004.
9. Hemostatic adhesive powder. Belozerskaya G.G., Malykhina L.S., Makarov V.A., Zhidkov E.A. Patent for invention: RUS 2304440 22.06.2005.
10. Hemostatic agent. Makarov V.A., Belozerskaya G.G., Momot A.P., Sokolov E.A., Ter-Arutyunyants A.A., Vasilyeva T.M. Patent for invention: RUS 2308287 26.12.2005.
11. Incomplete zinc salt of polyacrylic acid, a method for its preparation and an agent based on it, which has an antiseptic, hemostatic and wound healing effect when applied externally. Abzaeva K.A., Voronkov M.G., Zhilitskaya L.V., Fedorin A.Y.., Makarov V.A., Belozerskaya G.G., Zhidkov E.A., Fadeeva T.V., Kogan A.S., Grigoriev E.G. Patent for invention: RUS 2314815: 25.05.2006.
12. Synthetic derivatives of peptides. Belozerskaya G.G., Voyushina T.L., Makarov V.A., Malykhina L.S., Nevedrova O.E., Sergeev M.E., Sergeeva O.A., Ter-Arutyunyants A.A. Patent for the invention: RUS 2341532 12.10.2006
13. Textile material for stopping bleeding and a method for its production. Filatov V.N., Ryltsev V.V., Makarov V.A., Belozerskaya G.G. Patent for invention: RUS 2380117 26.09.2007.
14. An effective hemostatic agent based on lithium and double lithium-zinc salt of polyacrylic acid. Voronkov M.G., Abzaeva K.A., Zhilitskaya L.V., Makarov V.A., Belozerskaya G.G., Malykhina L.S., Malykhina S.G., Sergeeva O.A. Patent for invention: RUS 2424813 03.09.2008.
15. Incomplete rubidium salt of polyacrylic acid, method for its preparation and agent based on it, which has a hemostatic effect when applied externally. Abzaeva K.A., Voronkov M.G., Zhilitskaya L.V., Makarov V.A., Belozerskaya G.G., Malykhina L.S., Malykhina S.G., Sergeeva O.A. Patent for invention: RUS 2424814 02.10.2008.
16. Incomplete potassium salt of polyacrylic acid, a method for its preparation and an agent based on it, which has a hemostatic effect when applied externally. Abzaeva K.A., Voronkov M.G., Zhilitskaya L.V., Makarov V.A., Belozerskaya G.G., Malykhina L.S., Malykhina S.G., Sergeeva O.A. Patent for invention: RUS 2426546 02.10.2008.
17. Incomplete cesium salt of polyacrylic acid, a method for its preparation and an agent based on it, which has a hemostatic effect when applied externally. Abzaeva K.A., Voronkov M.G., Zhilitskaya L.V., Makarov V.A., Belozerskaya G.G., Malykhina L.S., Malykhina S.G., Sergeeva O.A. Patent for invention: RUS 2428989 02.10.2008.
18. Incomplete sodium salt of polyacrylic acid, a method for its preparation and an agent based on it, which has a hemostatic effect when applied externally. Abzaeva K.A., Voronkov M.G., Zhilitskaya L.V., Makarov V.A., Belozerskaya G.G., Malykhina L.S., Malykhina S.G., Sergeeva O.A. Patent for invention: RUS 2428990 02.10.2008.
19. Textile material for stopping bleeding and a method for its production. Filatov V.N., Ryltsev V.V., Makarov V.A., Belozerskaya G.G. Russian Federation patent for invention No. 2380117, 27.01. 2010, ballot No. 3.
20. Incomplete gold salt of polyacrylic acid, a method for its preparation and an agent based on it, which has a hemostatic effect when applied externally. Abzaeva K.A., Belozerskaya G.G., Malykhina L.S., Zhilitskaya L.V., Fedorin A.Y.., Bychichko D.Y., Lempert A.R. Russian Federation patent for invention No. 2607519 10.01.2017, ballot No. 1.
21. An effective hemostatic agent based on a double lithium-copper salt of polyacrylic acid, at the same time having a high antiseptic effect. Abzaeva K.A., Zhilitskaya L.V., Makarov V.A., Belozerskaya G.G., Malykhina L.S., Nevedrova O.E., Fadeeva T.V., Grigoriev E.G. Russian Federation patent for invention No. 2585366 20.12.2015 ballotNo. 35.
22. Hemostatic sponge and method for its production. Belozerskaya G.G., Makarov V.A., Momot A.P., Dzhulakyan U.L., Malykhina L.S., Nevedrova O.E., Bychichko D. Y., Lempert A.R., Golubev E M., Shirokova T. I., Shalnev D.V., Nikitina N.M., Kabak V. A., Logvinova Y.S., Mironov M. S. Patent of the Russian Federation for the invention No. 2628809 22.08.2017 ballot. No. 24.
23. Hemostatic sponge and method for its production. Belozerskaya G.G., Makarov V.A., Momot A.P., Dzhulakyan U.L., Malykhina L.S., Nevedrova O.E., Bychichko D.Y., Lempert A.R., Golubev E.M., Shirokova T.I., Shalnev D.V., Nikitina N.M., Kabak V.A., Logvinova Y.S., Mironov M.S. Patent of the Russian Federation for the invention No. 2618896 11.05.2017 ballot. No. 14.
24. Wound dressing with hemostatic effect, and method for its production. Savchenko V.G., Belozerskaya G.G., Makarov V.A., Malykhina L. S., Nevedrova O. E., Bychichko D.Y., Golubev E.M., Shirokova T.I., Shalnev D.V., Nikitina N.M., Kabak V.A., Momot A.P., Shakhmatov I.I.., Budaeva V.V., Gladysheva E.K., Skiba E.A., Sakovich G.V., Makarova E.I., Gismatulina Y.A., Bychin N.V. Patent of the Russian Federation for the invention No. 2624242 03.07.2017 ballot. No. 19.
25. Hemostatic sponge (variants). Belozerskaya G.G., Makarov V.A., Momot A.P., Dzhulakyan U.L., Malykhina L.S., Nevedrova O.E., Bychichko D.Y.., Lempert A.R., Golubev E.M., Shirokova T.I., Shalnev D.V., Nikitina N.M., Kabak V.A., Logvinova Y. S., Mironov M.S., Kuleshova S.B. Russian Federation Patent for Invention No. 2627855 14.08.2017 ballot. No. 23.
Studies to determine anticoagulant and antithrombotic activity are conducted by a leading researcher, Doctor of Biological Sciences N.N. Drozd.
In order to identify compounds with anticoagulant and antithrombotic activities, we study the structure-activity relationship, mechanisms of action, and pharmacological properties of new inhibitors of blood coagulation factors, new activators of plasma inhibitors of serine proteinases of the blood coagulation system, and new inhibitors of platelet aggregation. On the basis of our recommendations, co-authors-chemists have the opportunity to obtain active compounds in a targeted manner. An algorithm for a comprehensive assessment of the anticoagulant and antithrombotic activities of compounds in in vitro and ex vivo systems has been developed.
Research approaches and methods:
in vitro experiments:
- determination of inhibitory concentrations of compounds in standard coagulological tests blood coagulation time, activated blood recalcification time, plasma recalcification time, activated partial thromboplastin time, prothrombin time, thrombin time, ReaClot-Heparin (analogue of Heptest), ecarin (echitox) and reptilase (batroxobin) time with human and experimental animal plasma;
- determination of inhibitory concentrations of compounds in tests with chromogenic substrates for coagulation factors (including thrombin generation);
- determination of activity/concentration of primary physiological anticoagulants;
- determination of antithrombin and anti-factor Xa activity of compounds using International standards in accordance with the recommendations of USP, EP, the WHO International Standards Committee;
- assessment of the relationship between the structure and anticoagulant activity of compounds using regression, correlation and variance analyses;
- analysis of the antidote activity of the compounds in the neutralization of the activity of anticoagulants;
- analysis of complex formation with polycations (for polyanions) using biospecific electrophoresis or turbidimetric titration;
- analysis of the neutralization of the anticoagulant activity of polyanion compounds by polycation antidotes using coagulological and amidolytic tests;
- evaluation of the effect of compounds on platelet aggregation in humans and experimental animals induced by adenosine 5'-diphosphate, collagen, ristomycin;
- determination of platelet count;
- assessment of platelet adhesion
- assessment of erythrocyte hemolysis;
in vivo experiments:
- determination of pharmacodynamic parameters of compounds when administered intravenously, subcutaneously or orally to experimental animals (rabbits, guinea pigs) in comparison with anticoagulants or antiaggregants used in clinical practice;
- evaluation of the antithrombotic activity of compounds in the model of experimental venous thrombosis in guinea pigs according to S. Wessler;
- evaluation of hemorrhagic activity of compounds when administered to experimental animals.
Comparative studies of the specific anticoagulant activity of the following compounds obtained in the laboratories of co-authors-chemists were carried out:
- semi-synthetic polysaccharides of animal origin;
- chemically modified chitosans (A.N. Kosygin Moscow State Textile University and Bioengineering Center of the Russian Academy of Sciences);
- chitosan derivatives (I.Y. Postovsky Institute of Organic Synthesis, Ural Branch of the Russian Academy of Sciences, Yekaterinburg);
- quaternized chitosans (Bioengineering Center, Russian Academy of Sciences);
- native and semi-synthetic polysaccharides of plant origin;
- fucoidans from brown seaweeds of the Sea of Okhotsk Fucusevanescens, Laminariacichorioides, Laminariajaponica, Undariapinnatifida, Laminariagurjanovae, CostariaCostata (Pacific Institute of Bioorganic Chemistry, Far Eastern Branch of the Russian Academy of Sciences, Vladivostok);
- enzymatically digested fucoidans from the algae Laminariasaccharina; sulfated alginic acid from the brown algae Macrocystispurifera (Bioengineering Center of the Russian Academy of Sciences);
- chromatographically fractionated fucoidan from Laminariasaccharina algae (N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences);
- sulfated galactomannans from the seeds of CyamopsisTetragonoloba (L.) Taub., Galegaorientalis Lam, Styphnolobiumjaponicum, Locust Bean (A.N. Bach Institute of Biochemistry, Russian Academy of Sciences);
- chemically modified cellulose of cotton, fir, starch, inulin, pectin sulfates and conjugates of cellulose sulfates with terpenophenols (Institute of Chemistry, Komi Scientific Center, Ural Branch of the Russian Academy of Sciences, Syktyvkar);
- sulfated cellulose from fir, aspen, wheat straw, sulfated xylans, sulfated arabinogalactans and cyanidins from birch, spruce, pine, larch, and cedar bark (Institute of Chemistry and Chemical Technology, Siberian Branch of the Russian Academy of Sciences and the Siberian Federal University, Krasnoyarsk);
- pectin sulfates from herbaceous plants: bergenia crassifolia (L.) Fritsch., marsh cinquefoil (Comarumpalustre L.), duckweed (Lemnaminor L.), floating pondweed (Potamogetonnatans), common tansy (Tanacetum vulgare L.) (Institute of Physiology Komi Scientific Center of the Ural Branch of the Russian Academy of Sciences, Syktyvkar);
- nanoparticles based on chitosan and galactomannan loaded with low molecular weight or unfractionated heparins, polyelectrolyte complexes of chitosan sulfates, nanostructures based on alginic acid sulfate (Bioengineering Center of the Russian Academy of Sciences and A. N. Bach Institute of Biochemistry); chitin nanocrystals (Institute of Chemistry, Komi Scientific Center, Ural Branch, Russian Academy of Sciences, Syktyvkar);
- synthetic fucosylated oligosaccharides (N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences);
- synthetic derivatives of ZAla-Ala-Arg-Pip* TFA peptides; Z-Ala-Ala-Arg-Mf*HBr; Ac-Trp-Arg-Mf*HCl; Fta-Gly-Arg-Pip* TFA; Ac-Trp-Arg-Pip (State Research Institute of Genetics and Breeding of Industrial Microorganisms).
Patent protection (15 patents) is a prerequisite for the creation of new “atrombogenic” modifications of the surfaces of polymeric materials used in the manufacture of blood/plasma storage containers, vascular catheters, prosthetic blood vessels, blood clot traps, artificial heart valves, systems of cardiopulmonary bypass and auxiliary circulation systems etc.
The studied samples of polysaccharides with the highest antithrombin activity can be used as an independent coating on the surface of polymeric materials; substances with lower antithrombin activity can be conjugated with unfractionated or low molecular weight heparins (increasing their stability and activity), which are traditionally used to achieve "atrombogenicity" on the surface of some polymeric materials.
Quaternized chitosan, like protamine sulfate, completely neutralizes the antithrombin activity of unfractionated heparin and partially neutralizes the aXa activity of low molecular weight heparin (enoxaparin) when administered intravenously to guinea pigs.
The methodological base allows carrying out some studies necessary for the analysis of the hemocompatibility of water-soluble and water-insoluble substances and materials. Thus, dextran conjugates with 4-methyl-2,5-diisobornylphenol and chitin nanocrystals may be of interest as carriers for drug delivery.
A method for choosing an antidote for neutralizing the anticoagulant activity of sulfated polysaccharides based on the fixation of complexes between anticoagulants and polycations has been developed (RF Patent: 2370271).
Based on the received patents for inventions, (RF patent: 2221578 and RF patent: 2195673), sets of reagents for determining the activity of antithrombin in plasma and for determining the inhibition of fibrinogen-clotting and amidolytic activity of factor Xa by heparins have been created and are being produced.
PUBLICATIONS OVER THE LAST 5 YEARS
1. Drozd N.N., Shagdarova B.T., Il′ina A.V., Varlamov V.P.. Effects of Chitosan Derivative N-[(2-Hydroxy-3-Trimethylammonium)Propyl]Chloride on Anticoagulant Activity of Guinea Pig Plasma // Bull ExpBiol Med, 2017, V 163, N 3, p. 340-343, doi: 10.1007/s10517-017-3799-6.
2. Drozd N.N., Logvinova Y.S., Torlopov M.A., Udoratina E.V. Effect of sulfation and molecular weight on anticoagulant activity of dextran // Bull ExpBiol Med. 2017. V 162. No. 4. P 462-465; DOI: 10.1007/s10517-017-3640-2
3. Drozd NN, Logvinova YS, Torlopov MA, Udoratina EV. Effect of Sulfation and Molecular Weight on Anticoagulant Activity of Dextran // Bull ExpBiol Med, 2017, V 162, N 4, p. 462-465, doi: 10.1007/s10517-017-3640-2.
4. Drozd NN, Kuznetsova SA, Kalinina TB, Vasilieva NY. Dose Dependence of the Anticoagulant Effect of Intravenously Administered Cellulose Sulfate // Bull ExpBiol Med, 2016, V 160, N 6, p. 767-770, doi: 10.1007/s10517-016-3305-6.
5. Shagdarova BT, Il′ina AV, Varlamov VP, Drozd NN, Logvinova YS. Neutralization of anticoagulant activity of heparin by N-[(2-hydroxy-3-trimethylammonoium)propyl]chloride derivatives of chitosan // ApplBiochMicrobiol, 2016, V 52, N 4, P. 445-451, doi: 10.1134/S0003683816040141
6. S.A. Kuznetsova, N.N. Drozd, E.Y. Savchik, N.T. Miftakhova, N.Y. Antithrombotic agent from Siberian fir cellulose // RF Patent No. 2571555, publ. 20.12.2015
7. Drozd N.N., Shagdarova B.T., Ilyina A.V., Varlamov VP. Neutralization of the anticoagulant activity of guinea pig plasma with a quaternized derivative of chitosan.
8. Drozd NN, Savchik EY, Miftakhova NT, Kuznetsova SA, Vasilieva NY. Effects of subcutaneous microcrystalline cellulose sulfate extracted from wood of Siberian fir (abiessibiricaledeb) on the clotting of rabbit plasma // Pharm Chem J, 2015, V 49, N 3, P. 167-170. doi:10.1007/s11094-015-1246-4
9. Kuzhim A.A., Drozd N.N., Torlopov M.A.. Neutralization with protamine sulfate of the anticoagulant activity of sulfated cellulose isolated from Gossypium Hirstum L // Voprbiol med farm chemistry, 2014, M 12, N 1, pp. 34-38
10. Kuzhim AA, Drozd NN, Torlopov MA, Makarov VA. Neutralization with protamine sulfate of the anticoagulant activity of cellulose sulfates in vitro.
11. Drozd N.N., Kalinina T.S., Kuznetsova S.A., Vasil'eva N.Y., Kuznetsov B.N., Makarov V.A. Means with antithrombotic activity // RF Patent No. 2627435, publ. 08.08.2017.
12. Drozd N.N., Makarov V.A. Anticoagulant activity of substances, nano- and microstructures based on chitosan derivatives // In the book: Chitosan, ed. Skryabin K.G., Mikhailov S.N., Varlamov V.P., Moscow, 2013, pp. 530-563.
13. Kuzhim A.A., Drozd N.N., Torlopov M.A., Ilyina A.V. Relationship between the anticoagulant activity of sulfated plant polysaccharides and the size of the area of their precipitation with polycations during biospecific electrophoresis. 2013, T 76. 10, C 20-24
14. Savchk E.Y., Kalinina T.B., Drozd N.N., Makarov V.A., Zav'yalova E.G., Lapsheva E.N., Mudrik N.N., Babij A.V., Pavlova G.V., Golovin A.V., Kopylov A.M.. Aptamer RA36 inhibits of human, rabbit, and rat plasma coagulation activated with thrombin or snake venom coagulases // Bull ExpBiol Med, 2013, V 156, N 1, p. 44-48, doi: 10.1007/s10517-013-2274-2.
Participation in scientific conferences over the last 5 years
Poster reports
1. Kuzhim A.A., Drozd N.N., Torlopov M.A., Makarov V.A. / Influence of the degree of sulfation on the anticoagulant activity of starch and inulin // Congress of Hematologists of Russia, 2012, July 2-4, Moscow.
2. Savchik E.Y., Kalinina T.B., Drozd N.N., Makarov V.A., Zavyalova E.G., Lapsheva E.N., Babiy A.V., Mudrik N.N., Pavlova G.V., Golovin A.V., Kopylov A.M. / Analysis of the anticoagulant activity of Ra36 aptamer DNA using human, rabbit or rat plasma // Congress of Hematologists of Russia, 2012, July 2-4, Moscow.
3. Kuzhim A.A., Drozd N.N., Torlopov M.A., Makarov V.A. // Neutralization with protamine sulfate of the anticoagulant activity of cellulose sulfates in vitro // II Congress of Hematologists of Russia, 2014, April 17-19, Moscow.
4. Torlopov M.A., Udoratina E.V., Drozd N.N., Kuchin A.V. / Hemorheologically active sulfated derivatives of plant polysaccharides // III International Forum "BIOKIROV-2015" [Electronic resource]: Sat. Materials: September 17-19, 2015 Kirov. 2015. S. 20-22. ISBN 978-5-98228-084-8
5. Drozd N.N., Loginova Y.S., Torlopov M.A., Udoratina E.V. / Influence of sulfation on the anticoagulant activity of dextran // IX All-Russian scientific conference with international participation and the school of young scientists "Chemistry and technology of plant substances": Sat. Proceedings / Syktyvkar-Moscow. 2015. C. 53. ISBN 978-5-89606-542-5
Oral presentations
1. Neutralization of the anticoagulant activity of sulfated cellulose isolated from GossypiumHersutum with protamine sulfate in vitro and in vivo / Kuzhim A.A., Drozd N.N., Torlopov M.A. // Second Russian scientific and practical conference "Clinical and laboratory aspects of modern hematology" Theme of the conference "Physiology and pathology of the blood coagulation system", October 2013, Moscow
2. An antidote for heparins based on a chitosan derivative / Drozd N.N. // International conference "Modern perspectives in the study of chitin and chitosan" (RosHit-2016), September 5-10, 2016, Ufa.
3. Inactivation of heparin by quaternized derivatives of chitosan. B.T. Shagdarova, N.N. Drozd, A.V. Il'ina , Y.S. Logvinova , A.A. Zubareva , V.P. Varlamov // 8th International Conference “Biomaterials and nanobiomaterials: Safety-Toxicology And Ecology Issues” 07—14 May, 2017, Agapi Beach, Heraklion, Crete – Greece
Russian Foundation for Basic Research grants
97-04-48485-a Selective peptide inhibitors of thrombus formation and fibrinolysis. Development of their enzymatic synthesis;
02-04-49850-a Study of the relationship between the chemical structure and pharmacological activity of sulfated natural polysaccharides;
05-04-08100-ofi_a Influence of the structure of sulfated polysaccharides on their biological activity and the development of drugs for the prevention and treatment of thrombosis;
05-03-32883-a Study of the relationship between the chemical structure and biological activity of complexes based on natural heteropolysaccharides;
06-04-08140-ofi Investigation of the relationship between the structure of fucoidan polysaccharides isolated from seaweed and their anticoagulant activity in order to develop an anticoagulant agent;
07-04-12112-ofi Creation of a new antidote to neutralize the anticoagulant activity of sulfated polysaccharides based on chitosan and its derivatives;
Bid of the Ministry of Education and Science No. 02.434.11.3015 Code Лот-ЖС-12.2/003 High-performance methods for the search and synthesis of peptides and peptidomimetics - drug prototypes.
Contracts
Formalized preclinical studies "Anticoagulant activity of the synthetic aptamer Ra36" (Agreement No. НИР-19 dated 10.12.2010) were carried out in collaboration with Apto-Pharm LLC, the Faculty of Chemistry and the Faculty of Bioengineering and Bioinformatics of Moscow State University. M. V. Lomonosov and the Institute of Gene Biology- Russian Academy of Science.
The analysis of antithrombin and anti-factor Xa activities of several batches of generic low molecular weight heparin (solution for injection) was carried out in accordance with the regulatory documentation of a domestic pharmaceutical company, compiled according to the recommendations of the European and American Pharmacopoeia articles (Contract №0063-Д-ШФ dated 27.06.2011 .)
The work “Assessment of the effect of chitosan and its derivatives on some blood components” was carried out under Agreements No. 37 of 15.03.2016 and No. 48 of 02.05.2017 with the Federal State Institution “Federal Research Center “Fundamental Foundations of Biotechnology” of the Russian Academy of Sciences”.
Guidelines
Guidelines for the study of the anticoagulant activity of pharmacological substances have been developed and are included in the Guide for the experimental (preclinical) study of new pharmacological substances ["Guidelines for the study of pharmacological substances that affect hemostasis" p. 453-479 in the book. Guidelines for conducting preclinical studies of drugs. Part one / Ed. A.N. Mironova.- M.: Grif and K. 2012.- p. 944].

In the foreground: L.S. Malykhina. In the background, from left to right: O.E. Nevedrova, A.R. Lempert, G.G. Belozerskaya, M.S. Mironov, D.Y. Bychichko, V.A. Kabak, N.V. Drozd
KEY EMPLOYEES
Galina G. Belozerskaya — head of the laboratory, Doctor of Medical Sciences
Vladimir A. Makarov — scientific consultant, Doctor of Medical Sciences, Professor
Natalya N. Drozd — leading researcher, Doctor of Biological Sciences
Larisa S. Malykhina — senior researcher, Candidate of Biological Sciences
Olga E. Nevedrova — senior researcher, Candidate of Biological Sciences
Yulia S. Logvinova — biochemist, researcher
Dmitry Y. Bychichko — biochemist, junior researcher
Maxim S. Mironov - laboratory assistant
Asaf R. Lempert - trainee researcher
Valery A. Kabak - manager


